Management of Adult Snoring & Obstructive Sleep Apnoea
Table of Contents
Snoring
Snoring, defined as audible vibrations of the upper airway during respiration in sleep and affects up to 50% of the population.(1) Snoring has been associated with increased risk of carotid atherosclerosis, intima-media thickness(2,3) and metabolic syndrome(4) but not with cardiovascular disease, stroke or all cause mortality.(5) There are significant ramifications for the mental health and quality of life of bed partners of snorers with evidence demonstrating potential for chronic sleep deprivation, depression and fatigue.(6,7) Snoring does not always indicate OSA as demonstrated in the Sleep Heart Health Study which found that only two-thirds of participants with OSA reported snoring and over a third of habitual snorers did not have OSA (AHI ≤5).(8)
Obstructive sleep apnoea (OSA)
Obstructive sleep apnoea (OSA) is a condition characterised by upper airway obstruction during sleep, resulting in repetitive pauses in breathing termed apnoeas (complete reduction in airflow >90%) or hypopnoeas (reduction in airflow ≥30%) associated with oxygen desaturations and/or arousals from sleep. The latest epidemiological study estimates global prevalence of OSA at almost 1 billion with 425 million having moderate severity (AHI ≥15).(9) Obstructive sleep apnoea is common in patients with elevated BMI(10,11) and increasing age. (10–13) Therefore, the worldwide obesity epidemic and the ageing population demographic are likely to contribute to the continuing rise of obstructive sleep apnoea. It is estimated to affect 9% of women and 24% of men.(14) Moderate or severe OSA affects 20% of middle aged men and 10% of middle aged women in Australia.(15) Prevalence of moderate-to-severe sleep-disordered breathing (≥15 events per h) was 23.4% (95% CI 20.9–26.0) in women and 49.7% (46.6–52.8) in men in a community Swiss study.(16)
The apnoea–hypopnoea index (AHI) is a surrogate marker used to determine the presence and severity of OSA. It is calculated by the average number of apnoeas and hypopnoeas per hour of sleep and is classified as mild (5-15hr), moderate (15-30/hr) or severe (>30/hr).(17) However AHI poorly predicts the adverse outcomes of sleep apnoea whilst measures of hypoxaemia (low oxygen saturation) including the hypoxic burden (defined as the sum of individual areas under the oxygen desaturation curve) more strongly predicts cardiovascular disease and all cause mortality.(18)
Untreated, OSA is associated with a 5x increased risk of cardiovascular disease including hypertension, ischaemic stroke, coronary artery disease and heart failure, a 7 times risk of motor vehicle accidents, 2.6x risk of depression and insomnia and a >4x increased risk of all cause mortality. (19)(8)(20)
OSA is associated with a high economic and societal burden and In 2015, diagnosing and treating obstructive sleep apnoea in the USA cost approximately $12.4 billion.(21) The Australian community incurs an annual cost of $18.9 billion due to OSA, which encompasses direct health system expenses, secondary health issues, and productivity losses.(22)
Common risk factors for OSA include
- Gender: male 2x greater than women(10)
- Age: >65yrs 2x middle age(10)
- Family history
- Menopause (women)
- Obesity 2x rate than non obese(23)
- Anatomic
- Nasal obstruction
- Craniofacial abnormalities
- Tonsillar hypertrophy
- Macroglossia
- Sedatives: Alcohol, opioid or benzodiazepine consumption
- Medical history: thyroid disease, neuromuscular conditions
History
Ideally all consultations should be performed with the bed partner present. The snoring severity scale is used to determine if a patient snores for some, most or every night, if it can be heard for some, part or all the night and if it can be heard in the same room, next room or down the corridor.
Patients with obstructive sleep apnea (OSA) may be unaware of their condition and often seek help due to concerns raised by a bed partner or family member. Common signs reported by others include loud snoring, witnessed apneas, restless sleep, or choking episodes. It is important to record whether symptoms are influenced by sleeping position (eg. supine, lateral or prone). Patients might also experience frequent awakenings, nocturia, or difficulty staying asleep. Daytime symptoms include feeling unrefreshed upon waking, excessive sleepiness, frequent napping, poor concentration, mood changes, and fatigue. Some may seek help after an accident caused by sleepiness. Men often present with classic symptoms like snoring, apneas, and daytime sleepiness, while women may report more general symptoms like fatigue, depression, or insomnia.(24) Approximately 30–50% of patients with OSA report clinically significant insomnia symptoms.(25) Comorbid insomnia and OSA (COMISA) results in greater morbidity including excessive daytime sleepiness (EDS), depression and potential mortality.(26)
EDS can also result from other factors, such as insufficient sleep due to work or social demands. Other medical conditions, including anemia, diabetes, hypothyroidism, mood disorders, or medications, can also cause fatigue and require investigation and management as indicated. Sleep disorders like narcolepsy or idiopathic hypersomnia, and conditions like delayed sleep phase syndrome, can present with similar symptoms of daytime drowsiness and often require sleep physician review.
While there are some similarities in symptoms between children and adults, it is crucial to recognise that the aetiology, classification, and presentation often differ. In children, it is important to consider inattention, irritability, and behavioural and learning problems. For further information, please refer to my previous article.
Examination
Physical examination should include assessment of body mass index (BMI), waist circumference (WC) and neck circumference along with blood pressure measurement. An increased risk of OSA is associated with BMI ≥30 kg/m2; WC male ≥102 cm, female ≥88 cm; neck circumference male ≥42 cm, female ≥39 cm.
A comprehensive ear nose and throat examination includes craniofacial assessment with increased risk of OSA associated with retrognathia, micrognathia, maxillary hypoplasia. Dentition and occlusion is inspected to determine potential suitability for oral appliance therapy. Hard palate and Friedman Tongue Position (similar to Mallampati but with tongue in) are evaluated with narrow high arched palate and grade 3-4 Friedman Tongue Position associated with worse surgical outcomes whilst conversely tonsil hypertrophy is associated with better post operative outcomes.
Anterior rhinoscopy is performed to detect reduced nasal patency resulting from septal deviation, nasal valve collapse, turbinate hypertrophy, nasal polyps or rhinitis.
Flexible nasendoscopy involves passing a 2-3mm endoscope intranasally to assess the nasal passages, post nasal space for tumours, determine soft palate configuration (vertical less likely to respond as well to surgery) and perform awake dynamic assessment with volitional snore(27), Müller manouvre(28)Following a forced exhalation, an attempt to inhale is made with the mouth and nose closed, creating a significantly subatmospheric pressure in the chest and lungs; this is the opposite of a Valsalva maneuver. and Woodson’s hypotonic method(29). Both techniques are useful to determine the site (retropalatal, oropharyngeal and retrolingual) and pattern (anteroposterior, lateral, concentric) of upper airway collapse. These manouvers are then repeated with maximum jaw protrusion to see the potential impact oral appliance therapy has on the anatomy with responders demonstrating greater naso- and oropharyngeal anterior tongue movement as compared to nonresponders.(30)
Questionnaires
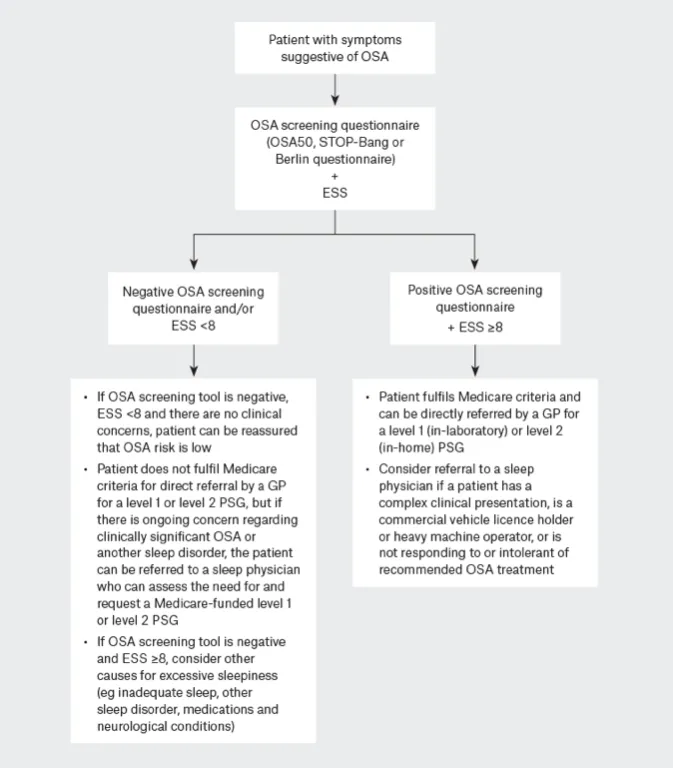
OSA screening questionnaires like OSA50 (31). STOP-Bang (32)and Berlin(33) to identify patients at high risk for OSA. These questionnaires have similar diagnostic accuracy for moderate–severe OSA and are highly sensitive but have low specificity, leading to high false positive rates. Therefore, a positive result requires further sleep study testing. The OSA50 is often preferred for its simplicity and being specifically developed for an Australian primary care population.
Investigations
Polysomnogram (sleep study)
There are 4 levels (see below) of sleep study with Medicare rebates available for level 1 or 2 full PSG but not levels 3 or 4 limited-channel sleep studies. As the level decreases the diagnostic accuracy and ability to detect OSA and other sleep disorders diminishes.(34)
Level 1: Laboratory-based, full polysomnography (PSG) with ≥7 recording channels that includes measurement of sleep – the gold standard
Level 2: Unattended, full PSG with ≥7 recording channels, usually conducted in the patient’s own home
Level 3: Limited cardiorespiratory recording (4–6 channels), without measurement of sleep
Level 4: Limited recording with only 1–3 channels (including oximetry), without measurement of sleep
The WatchPAT device (Itamar Medical, Ltd.) is a wrist-worn sleep study tool that uses peripheral arterial tonometry (PAT), pulse oximetry, and actigraphy to detect respiratory disturbances like apnoea and hypopnoea. It analyses changes in peripheral arterial volume, oxygen saturation, and heart rate using an automated algorithm to assess respiratory effort-related arousals. While convenient and less invasive than polysomnography (PSG), it estimates sleep stages less precisely, may under-detect hypopneas without oxygen desaturation, and can overestimate REM sleep.
A 2022 meta-analysis(35) of 17 studies (1,300+ patients) found WatchPAT showed high sensitivity but variable specificity for detecting obstructive sleep apnoea (OSA) at different severity thresholds. It is not Medicare-rebated and is contraindicated in conditions such as central sleep apnoea, neuromuscular disease, and congestive heart failure. For these reasons, it should not replace PSG for complex sleep disorders or definitive OSA diagnosis.
Investigation pathway
Motor vehicle accidents
Motor Vehicle Accidents (MVAs) are a leading cause of morbidity and mortality with approximately 1.19 million deaths globally each ear. Sleepiness at the wheel (SW) results in impairment of drivers’ reaction time, vigilance, and decision‐making and increases the odds of an MVA by 2.5 and accounts for 10-30% of fatal MVAs.(89,90) OSA is a well established risk factor for SW and MVA.(90)
In 2025 a retrospective cohort study was published comparing the outcomes of MVA in patients with OSA treated with CPAP or surgery.(91) A total of 2,832,437 patients with OSA were identified. Patients with OSA undergoing sleep surgery demonstrated a significantly lower incidence of MVAs (3.403%) compared to the OSA+CPAP cohort (6.072%) and the no-treatment group (4.662%). The odds ratio (OR) for MVA incidence in the no-treatment group compared to the OSA + sleep surgery cohort was 1.214 (95% confidence interval [CI]: 1.060-1.391, P = .0051). The OR in the OSA + sleep surgery cohort compared to the CPAP cohort was 0.545 (95% CI: 0.480- 0.618, P < .0001). Patients with OSA who experienced MVAs were more likely to have comorbidities such as hypertension (58.8% vs 45.4%), obesity (42.3% vs 34.7%), and diabetes (33.8% vs 24.2%) following the accident.
Conclusion. Sleep surgery significantly reduces the risk of MVAs in patients with OSA compared to CPAP and no treatment. In appropriate candidates, surgery should be considered to mitigate personal and public health risks associated with OSA
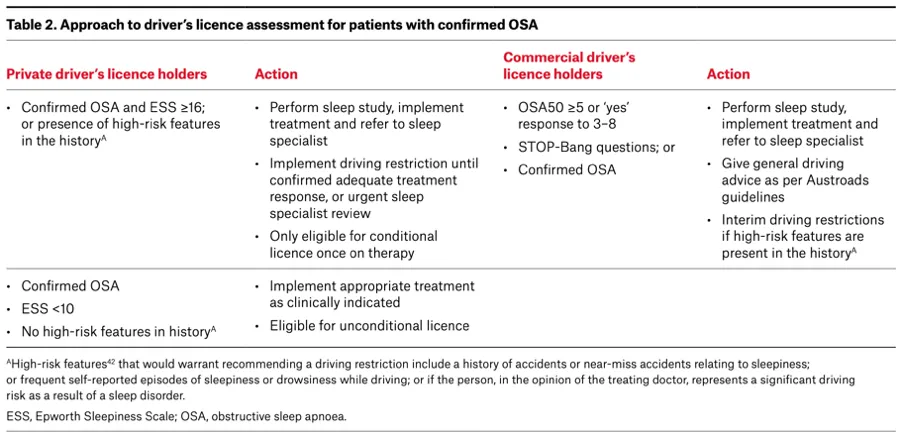
Commercial drivers license
Commercial drivers have a higher prevalence of OSA than the general population. In a study of 3,268 commercial truck drivers in Australia, with similar proportion of men and higher proportion of responders, 60% had OSA confirmed by PSG. Further, 24% reported excessive sleepiness, which was related to increased crash risk.(92)
Commercial drivers in Australia require referral to a sleep physician and those who require treatment must have an annual review by a sleep physician to ensure symptom control and adequate management of OSA including compliance with device therapy.(93) CPAP machine should have a usage metre to objectively assess and record treatment compliance – ≥4h per day, 70% of days.(94). If a patient has had multi level upper airway surgery and is cured of OSA as demonstrated by PSG and does not have symptoms of sleepiness then they do not require further review unless symptoms of OSA and sleepiness return. Physcians must assess sleepiness and consider objective measurement of sleepiness (MWT) if concern about persisting sleepiness or treatment compliance.
For further information see Table 2 (below) from the article published by Ellender and colleagues in 2024 which outlines approach for license assessment in private and commercial drivers. (57)
Referral to a sleep physician should be considered if
- Symptoms suggestive of another disorder affecting sleep (e.g., insomnia, parasomnia, narcolepsy, or periodic limb movement disorder)
- Complex clinical presentation or no improvement with standard OSA therapies
- Sleepiness-related motor vehicle or work accident
- High occupational risk
- Commercial vehicle licence holder
- Pilots
- Heavy machinery operators
- BMI ≥45kg/m2
- Alcohol or Chronic opioid use
- Neuromuscular disease
- Significant respiratory disease (e.g., severe COPD)
- Heart failure
Treatment
Conservative
Weight Loss:
- Significantly reduces snoring, AHI, and excessive daytime sleepiness (EDS)(36)
- A 10% weight loss can cause a clinically meaningful reduction in AHI(37)
- Every kilogram of weight lost results in a reduction in AHI of 0.78 events/hour(38,39)
- Long-term benefits include cardiovascular risk reduction
Dietary and Exercise Interventions:
- Improve sleep quality and arousal threshold independent of BMI reduction(40)
Alcohol Reduction:
- In a 2020 meta-analysis(41) alcohol consumption was associated with worsening severity of snoring, altered sleep architecture, AHI, as well as lowest oxygen saturation among patients susceptible to snoring and obstructive sleep apnoea. A mean difference of 3.98 AHI and -2.72% was noted between alcohol and control for AHI and LSAT respectively.
GLP-1 Agonists:
- SURMOUNT OSA(42) was a phase 3 double blinded randomised controlled trial involving 469 non diabetic adults with obesity and OSA separated into those with and without CPAP comparing Mounjaro (tirzepatide) a combined incretin GLP-1 plus glucose dependent insulinotropic polypeptide (GIP) agonist to placebo. It demonstrated amongst adults with moderate-to-severe obstructive sleep apnea and obesity, tirzepatide reduced the AHI by 27.4/hr compared to 4.8/hr placebo (Non CPAP group) and 29.3/hr versus 5.5/hr (CPAP group). Body weight was reduced by 17.7 and 19.6% for Non CPAP and CPAP groups respectively. Reduction in Hypoxic burden, hsCRP concentration, and systolic blood pressure and improved sleep-related patient-reported outcomes was also demonstrated.
- TGA has approved tirzepatide for the treatment of overweight and obesity for those with comorbidities, via private prescription. It is currently FDA approved for treatment of OSA in the United States and may be incorporated into international guidelines in future.
COMISA
- It is important to recognise presence of COMISA. Recent RCT evidence demonstrates that initial treatment of insomnia with cognitive behavioural therapy improves insomnia symptoms and might increase CPAP compliance and adherence.(43)
Device therapy
CPAP is a compact bedside device that uses positive pressure to keep the upper airway open and prevent it from collapsing. The machine connects to the face via a hose and a mask, which can be nasal, nasal pillow, full face or an over-the-mouth hybrid design. Positive pressure can be delivered through four main types or algorithms: continuous (CPAP), auto-titrating/APAP (auto-titrating positive airway pressure [APAP]), adaptive servo-ventilation (ASV), or bilevel positive airway pressure (BiPAP). APAP is a type of CPAP that uses sensors to adjust the pressure as needed to maintain an open airway and is often used to initiate PAP therapy in uncomplicated OSA and in most instances patients can be switched to CPAP. BIPAP alternates between inspiratory and expiratory pressure and typically used for treating hypoventilation disorders or if very high pressures are required. ASV are ventilators used for treatment of central sleep apnoea and require sleep physician oversight.
Multiple international guidelines (44,45) recommend CPAP as the first-line treatment for most adults with symptomatic moderate-to-severe OSA or mild OSA with significant hypoxemia and/or EDS. There is high-grade evidence that CPAP improves AHI and moderate-to-high-grade evidence for improvements in ESS and quality of life. Other potential benefits include improved anxiety and depression symptoms, motor vehicle crash risk, atrial fibrillation recurrence and blood pressure control. However to date randomised control trial data has not shown any cardiovascular secondary prevention benefits (cardiovascular events or mortality) associated with CPAP treatment in adults with moderate-to-severe OSA and minimal sleepiness.(46–48)
CPAP when deliberately titrated in OSA to eliminate snoring has been shown to be effective and safe whilst in non-apnoeic snorers, low CPAP levels of 4–6 cm H2O have been proven to be effective in eliminating snoring.(49)
However, OSA treatment benefits are dose dependent, with four hours per night of CPAP recommended across populations. However in a study of almost 2000 patients CPAP usage times per night to normalize the AHI values were 3.3 h, 5.6 h and 6.5 h for mild, moderate and severe OSA categories, respectively.(50) Consequently many moderate to severe OSA patients are not being adequately treated with CPAP resulting in a significant residual AHI, which could explain why some clinical trials fail to show significant benefits. However non adherence to CPAP has been reported between 29 to 83%.(51–54) The overall CPAP non-adherence (<7h/night) rate across 82 studies over a 20 year period was reported as 34.1% with no clinically significant improvement in CPAP adherence over time despite efforts toward behavioural intervention and patient coaching.(55)
Long term CPAP adherence data demonstrates less than 4 hours per night of CPAP amongst patients in trial setting after 12 months, with the first month was the most predictive of adherence at 24 months.(56)
The cost of self-funding a CPAP machine varies among retailers, from $900 to $2000. Many private health funds will rebate between $500 and $1000 for set-up costs. Masks generally require replacement every three years, with silicon mask cushions and head straps requiring replacement approximately every 12 months depending on cleaning routines.(57)
Oral appliances
Oral Appliance Therapy (OAT), most commonly a Mandibular Advancement Splint/Device (MAS or MAD) helps treat obstructive sleep apnoea (OSA) by repositioning the lower jaw (mandible) forward and downward. This action increases the size of the airway by pulling the tongue forward (58) (genioglossus) and to a lesser extent, the soft palate (palatoglossus and pharyngeal constructor muscles).(59–61) The increase in the airway size occurs in both the front-to-back (antero-posterior and side-to-side (lateral) directions, with the greatest expansion observed in the lateral dimension in the velopharynx in one study.(60)
The American Academy of Sleep Medicine and the American Academy of Dental Sleep Medicine 2015(62)and 2021 NICE guideline (44) recommend that OAT should be offered to adult patients with OSA who are unable to tolerate or declined CPAP. OAT should be custom made by a dentist qualified and experienced in dental sleep medicine and who preferably holds a Fellowship of Dental Sleep Medicine postgraduate qualification.
The ORCADES study evaluated the long-term effectiveness of OAT for managing obstructive sleep apnoea (OSA) in patients who were either intolerant of or refused continuous positive airway pressure (CPAP). At the 5-year follow-up, data was available for 172 of an initial 331 patients with treatment success (defined by a ≥50% reduction in AHI) achieved in 52% of patients overall, with the highest success rates seen in those with moderate to severe OSA.(63)
A crossover trial compared the effectiveness of OAT compared to CPAP on quality of life outcomes and snoring. OAT therapy significantly reduced snoring outcome snores with no difference between the two groups. (64) In a randomized crossover trial comparing a custom-made titratable MAD to a thermoplastic, or “boil and bite” MAD, the custom-made device was more effective in reducing the AHI and controlling snoring. The thermoplastic device, on the other hand, had no effect on AHI.(65)
Frequently reported shot term side effects related to custom-fitted devices include excess salivation, dry mouth, morning after occlusal changes, difficult chewing and discomfort in dentition, gums or TMJ. Long term side-effects (progressive and irreversible) are rare typically involve tooth movement and occlusal change. Duration of appliance use and the amount of mandibular advancement linked to magnitude of side-effects.(66)
OAT (e.g. MAS/MADs) is not funded by Medicare. Concession/Health Care Card holders may access devices via public dental services, but with long wait times and co-payments of $400–$900. Most patients require private dental referral, with out-of-pocket costs of $1200–$2100. Devices typically last ≥4 years, depending on type, brand, and maintenance. Private health funds may rebate $300–$1000 under extras cover.(57)
Position Dependant OSA (POSA)
In approximately 56% to 75% of patients with obstructive sleep apnoea (OSA), the frequency and duration of apnoea’s are influenced by body position.(67) Position Dependant OSA (POSA) is defined by an AHI ≥ 15 events/h, supine which is twice the nonsupine AHI, with ≥ 20 minutes of sleep in supine and non supine postures, and nonsupine AHI < 15.(68) The majority (70-80%) have only mild or moderate OSA.(67) Patients with POSA can be treated with a small device attached to either the neck or chest which detect when patients are supine and provide a vibrating stimulus until the patient returns to a lateral position. Positional therapy has been shown to reduce the AHI by 11.3events/h (54% reduction) and 33.6% of total sleep time (84% reduction) in a recent systematic review and meta-analysis.(67) In a prospective multicentre randomized crossover trial treatment with PT resulted in non-inferior treatment efficacy and greater adherence compared to APAP in patients exclusive POSA.(69) Costs in Australia vary from $150 to $400 depending on the type of device.(57)
Nasal obstruction
The nose accounts for 50% of upper airway resistance and when obstructed there is a physiological switch to oral breathing.(70,71) Oral breathing modifies upper airway dynamics displacing the mandible, tongue, soft palate posteriorly reducing the calibre of the upper airway.(72) It is associated with an up to 2 fold higher airway resistance and in a randomised study of a healthy population led to significantly more frequent obstructive (but not central) apnoeas and hypopnoeas increasing the AHI measured on polysomnography compared to nasal breathing.(73) Nasal obstruction is present in almost 30% of Europeans with the leading causes including Allergic Rhinitis (AR) and Chronic Rhinosinusitis (CRS).(74) CRS and AR have both demonstrated a negative impact on sleep quality and objective parameters in sleep studies.(75,76)
CPAP adherence is estimated at approximately 50% demonstrating a significant reduction in long term follow up according to several studies.(77–80) A major factor of poor adherence is related to the mask of which nasal symptoms reported by 30% to 50% of the patients are the first cause of lack of adherence.(81) High nasal resistance, as objectively measured through rhinomanometry, has been statistically correlated as a predictor of low adherence to CPAP.(82,83)
Treatment of nasal obstruction improves sleep architecture, reduces mouth breathing during sleep and obstructive sleep apnoea severity.(84) Endoscopic Sinus surgery has been shown to improve sleep quality in CRS patients.(85) In a 2015 meta-analysis of 18 studies (279 patients) demonstrated a statistically significant mean CPAP pressure reduction (in 7 studies with 82 patients) of -2.66 centimetres of water pressure. In the same analysis eleven studies (153 patients) described subjective, self- reported data for CPAP demonstrated that 89.1% (57 of 64 patients) who were not using CPAP prior to nasal surgery subsequently accepted, adhered to, or tolerated it after nasal surgery. Objective, device meter-based hours of use increased in 33 patients from 3.0 ± 3.1 to 5.5 ± 2.0 h in the short term (< 6 mo of follow-up).(86) Due to the poor adherence rate of CPAP appropriate evaluation and treatment of the nasal airway should be completed before initiating PAP therapy. Nasal surgery should be offered to all patients with anatomical obstruction and suboptimal use of CPAP or high titration pressures.(72)
Conservative measures
The evidence strongly supports the use of nasal steroids for nasal obstruction in a variety of conditions, particularly allergic rhinitis, non-allergic rhinitis, and chronic rhinosinusitis.
Allergic Rhinitis: The American College of Allergy, Asthma, and Immunology (ACAAI), the American Academy of Otolaryngology and ARIA (Allergic Rhinitis and its Impact on Asthma) guidelines recommend nasal corticosteroids as first-line treatment for nasal obstruction in allergic rhinitis with clear evidence of their efficacy in clinical trials. A large RCT published in JAMA (2015) demonstrated significant symptom improvement in patients with moderate-to-severe rhinitis after treatment with fluticasone furoate nasal spray, highlighting the benefit in reducing nasal congestion and improving quality of life. Their effectiveness in reducing nasal swelling, improving nasal airflow, and providing long-term symptom control makes them the cornerstone of therapy for these conditions. Their safety profile, when used as directed, further reinforces their role as a first-line treatment for nasal obstruction.
When to refer to OSA trained ENT surgeon?
The American Academy of Sleep Medicine (87)recommends referral to an appropriately trained Sleep surgeon for
- Patients who are intolerant or unaccepting of PAP therapy
- Patients who have persistent inadequate PAP adherence due to pressure-related side effects
- Patients with obvious upper airway anatomic abnormalities potentially amenable to surgery as initial OSA treatment
Referral to a sleep trained ENT surgeon should be considered if
- Presence of nasal obstruction or snoring
- High clinical suspicion for OSA despite a negative response to an OSA screening questionnaire and/or an ESS score <8
“Sleep surgeon” refers to an otolaryngologist or oral and maxillofacial surgeon with training and expertise in upper airway surgery who has an appropriate understanding of sleep medicine and modern surgical techniques for the treatment of OSA
Multilevel upper airway surgery for snoring
In a retrospective case series 68 adults including 17 females with primary snoring who underwent modified uvulopalatopharyngoplasty and coblation tongue channelling. Modified UPPP with RFS tongue treatment significantly reduced snoring severity and daytime sleepiness in patients with primary snoring. The mean SSS prior to surgery was 7.0±1.6 and 1.9±2.3 whilst the mean ESS prior to surgery was 9.0±4.8 and 4.1±2.8 at 3-month follow-up. This procedure, with a low complication rate, is an effective option for patients who have not benefited from or declined other therapies, such as CPAP or oral appliances.(88)
UPPP + Coblation tongue channelling
The Sleep Apnea Multi-level Surgery (SAMS) randomized clinical trial(18) hypothesized that multilevel surgery in adults might be better than ongoing medical management when CPAP fails. Patients with OSA not able to tolerate CPAP (or other devices) were included in the study and were randomized to receive either surgery with modified Uvulopalatopharyngoplasty (UPPP) and radiofrequency tongue channelling or best medical management. Best medical management included weight loss and alcohol reduction, sleep positioning, management of nasal obstruction or other lifestyle measures. Fifty-one patients were enrolled in each treatment arm. There was a statistically significant improvement from baseline in the surgical group compared to medical group in both co-primary outcomes of AHI (events/hr) and Epworth Sleepiness Scale (ESS). Surgery resulted in a mean AHI reduction of -27(events/hr) compared with -10(events/hr) for medical management whilst the mean ESS was 12.4 at baseline and 5.3 at 6 months following surgery compared with minimal change of 11.1 at baseline and 10.5 at follow up with medical management, see Figure 1. Compared with participants with ongoing medical management, surgery participants were noted to have improved sleep quality represented by lower frequency of arousals (mean 33 to 19/hour), improved sleep oxygenation (mean 3% oxygen desaturation index change from 25 to 7/hour)) and time spent below 90% oxygen saturation (mean 8.8% to 3.8%). At 6 months there were statistically significant mean between group differences favouring surgery for partner-reported snoring (Snoring Severity Index -4.0, 95% CI, -4.9 to -3.1), sleep specific quality of life (Functional Outcomes of Sleep questionnaire score 3.4, 95% CI 2.5 to 4.4) and specific health benefits (Glasgow Benefit Inventory 32.1 (22.8)). The Body Mass Index (BMI) was 30.7 (versus 29.5 in medical group) and did not change significantly between the pretreatment and post treatment assessment points in both groups, countering a common misconception of improvement from multi-level surgery due to weight loss. Long term outcomes (mean 3.5 years) were recently published (19) which demonstrated maintenance of treatment effect for multi-level upper airway surgery in adults with moderate or severe OSA in whom conventional therapy failed. Of the surgical participants who were re-evaluated using polysomnography, AHI was 41/h at preoperative baseline and 21/h at follow up representing durable improvement of -24/h (95%CI -32 to -17, p< 0.001) whilst ESS was reduced by -6.8 (12.3 at baseline reduced to 5.5). Secondary outcomes remained improved and of the 84% of the medical management participants who were re-evaluated, 25 (69%) underwent subsequent surgery and reported overall satisfaction and improved quality of life.
Lingual tonsil hypertrophy and lingual tonsillectomy
Lingual tonsils are situated at the base of tongue and bounded by the epiglottis posteriorly, circumvallate papillae anteriorly, and tonsillar pillars bilaterally. They are a component Waldeyer’s ring along with the palatine tonsils, adenoids, tubal tonsils, and lateral pharyngeal bands.(95) Lingual Tonsillar Hypertrophy (LTH) is most commonly caused by previous adenotonsillectomy, obesity and laryngopharyngeal reflux in both children and adults.(96–98) LTH is a common cause of base of tongue obstruction which was a site of collapse in 46% of over 1000 patients with OSA who underwent DISE in a study published in the laryngoscope in 2013.(99) In a 2018 systematic review and meta-analysis(100) of four studies (107 patients) who underwent lingual tonsillectomy and palate surgery demonstrated a statistically significant average AHI reduction of 18.51 events/h, increase in minimal arterial oxygen saturation of 5.26 and average Epworth Sleepiness Scale (ESS) reduction of 5.44. In a retrospective analysis published in 2019 by Lan and colleagues isolated lingual tonsillectomy comparing TORS versus Coblation assisted for treatment of patients with OSA and LTH. The AHI (mean ± SD) reduced significantly from 50.5 ± 19.6 events/h to 25.5 ± 19.5 events/h in the TORS group (p = .002). In the coblation group, the mean AHI reduced significantly from 44.8 ± 28.8 events/h to 25.5 ± 23.3 events/h (p = .005). The AHI reduction in the TORS and coblation groups were significantly reduced, and the between-group difference was not significant (p = .631; 95% CI [−12.67–23.73], Fig. 3 and 4). The mean improvement of min SpO2 was 10.0 ± 7.7% in the TORS group and 6.7 ± 12.6% in the coblation group. There were no statistically significant differences in the improvement of min-SpO2 between the two groups.
Transpalatal advancement pharyngoplasty
In a 2016 SR and MA evaluating Transpalatal Advancement Pharyngoplasty (TPA) five studies met criteria with 199 patients (age: 42.5 ± 9.7 years and body mass index: 29.0 ± 4.0 kg/m2). The grand mean (M) and standard deviation (SD) for AHI (199 patients) pre and post-TPA decreased from 54.6 ± 23.0[95 %CI51.4,57.8] to19.2 ± 16.8[95 %CI 16.9, 21.5] events/h (relative reduction: 64.8 %). Total AHI reduction was 35.4
LSAT (measured in 70 patients), the pre and post-TPAP M ± SD improved from 81.9 ± 8.1 [95 % CI 80.0, 83.8] to 85.4 ± 6.9 [95 % CI 83.8, 87.0], with a MD of 3.55, overall effect Z = 1.79 (p = 0.07)
Midline glossectomy
In a 2015 SR and MA(101) evaluating glossectomy (midline glossectomy, lingualplasty, and submucosal minimally invasive lingual excision – SMILE) as part of multi-level upper airway surgery 18 articles with 522 patients were identified. Pooled analysis demonstrated a significant reduction in AHI (-27.81 events/h), ESS (-5.49), snoring (-5.6 visual analogue scale) and improvement in LSAT (+7.68). Complications occurred in 16.4% of patients most commonly taste change reported in 5.8% that within 2 months. Post operative bleeding occurring in 4.2%, temporary dysphagia and globus whilst temporary hypoglossal nerve neuropraxia <1% and one case of permanent injury.
Midline glossectomy
In a 2015 SR and MA(101) evaluating glossectomy (midline glossectomy, lingualplasty, and submucosal minimally invasive lingual excision – SMILE) as part of multi-level upper airway surgery 18 articles with 522 patients were identified. Pooled analysis demonstrated a significant reduction in AHI (-27.81 events/h), ESS (-5.49), snoring (-5.6 visual analogue scale) and improvement in LSAT (+7.68). Complications occurred in 16.4% of patients most commonly taste change reported in 5.8% that within 2 months. Post operative bleeding occurring in 4.2%, temporary dysphagia and globus whilst temporary hypoglossal nerve neuropraxia <1% and one case of permanent injury.
Cranial nerve stimulation
There are various nerve targets for stimulation in the treatment of obstructive sleep apnoea. Currently hypoglossal nerve stimulation (HGN) is the most common type, most extensive studied and available in the US, Europe and many other parts of the world with different commercial manufactures. Since its approval in 2014 by the US FDA over 50,000 patients in the US have been implanted. HGN works by selectively stimulating branches of the hypoglossal nerve such as the genioglossus which when activated (whilst the patient is asleep) protrude the tongue forward to alleviate retro lingual (base of tongue obstruction). In numerous studies including randomised control trials and long term (Inspire) (5 year follow up) it has been shown to normalise Epworth Sleepiness Scale scores in 80% and significantly reduce AHI by >68% with improvements in oxygen saturation and other sleep parameters.(102)
The ADHERE Registry is a multicentre prospective observational study following outcomes of upper airway stimulation (UAS) therapy in patients who have failed continuous positive airway pressure therapy for obstructive sleep apnoea (OSA). At the time of the 2020 update(103)1,849 patients had enrolled in ADHERE and 1,019 reached final visit with 843 completed the visit. Significant changes in AHI (20.9, P < .0001) and ESS ( 4.4, P < .0001) were demonstrated. Mean therapy usage was 5.6 2.2 hr/day. Significant therapy use difference was present in patients with reported discomfort versus no discomfort (4.9 2.5 vs. 5.7 2.1 hr/day, P = .01). Serious adverse events reported in 2.3% of patients. Device revision rate was 1.9%. Surgical success was less likely in BMI35 versus BMI32 patients (59.8% vs. 72.2%, P = .02). There was a significant therapy use difference: 5.8 2.0 hr/day in BMI32 versus 5.2 2.2 hr/day in BMI35 (P = .028).
A large multicentre RCT(104)of 138 patients with moderate to severe OSA (AHI 20 to 65) and BMI ≤35 underwent Implant with THN (LivaNova) system; randomized 2:1 to activation at month 1 (treatment) or month 4 (control). All received 11 months of THN with follow-up at months 12 and 15, respectively.
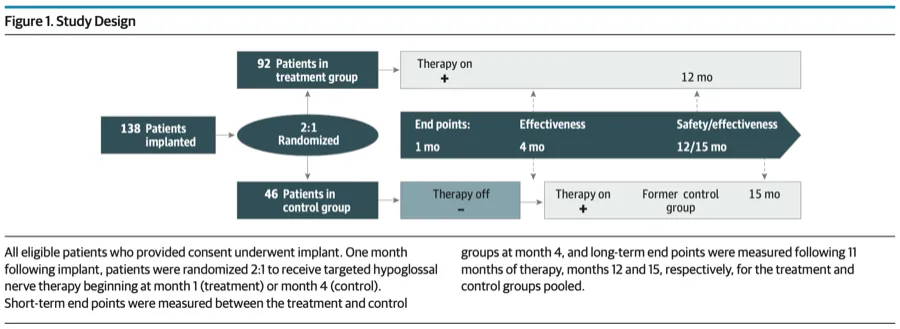
THN therapy significantly improved measures of sleep-disordered breathing and patient-reported outcomes, including daytime somnolence and quality of life in treated vs control participants.
In the 2020 update of the ADHERE registry(105) 1017 patients had been enrolled and 382 completed the 12 month follow-up with median and mean AHI reduction of 23.3 and 21.6 events/h respectively. ESS improved by a median and mean of 5 and 5.2 respectively. Therapy usage was 5.6 2.1 hours per night after 12 months. Stimulation-related discomfort was the most common complaint reported by 8% of participants at 12 months post-implantation. Infection occurred in 2 (<1%), swallow disturbance occurred in 4 (1%) and tongue weakness in 3 (<1%) of patients with all bar one patient with persisting swallow disturbance resolving at 12 months. Surgical intervention was required for device revision in three cases: in one participant due to stimulation electrode dislodgement within 6 months and in another two participants with stimulation electrode repositioning within 12 months.
It is currently approved by the TGA in Australia but not funded by Medicare which limits its application to clinical trials. – I have been involved in a first in human study in 2023 and will be involved in a new hypoglossal nerve stimulation device later this year with a US based HGN company.
Hypoglossal nerve stimulation has been recently approved for paediatric patients with down syndrome and other complex medical conditions with less than 100 paediatric patients implanted worldwide in the literature. All studies have been retrospective thus far but with evidence of improving QOL, reduction in AHI, improvement in oxygen saturation and parent/care giver satisfaction.(106,107) I was involved in a panel on this very topic at the 2024 International Sleep Surgical Society conference and there are several paediatric specific challenges limiting its broader use and future studies are required.
Ansa cervicalis stimulation is the second most studied target for treatment of OSA however it is still in clinical trial stage and not been approved or commercialised for use. It acts to “tension and stiffen” the airway making it less collapsable which improves airway flow.(108)
Vagal nerve stimulation is currently used for treatment of certain neurological, anti-seizure treatment and psychological conditions however has been associated with causing OSA in up to 30% of cases.(109–111)
Bariatric surgery
The American Thoracic Society (112) and the American Academy of Sleep Medicine (87) recommend bariatric surgery for adults with moderate-to-severe obstructive sleep aponea (OSA) and a BMI over 35 kg/m² who cannot tolerate CPAP therapy. Bariatric surgery improves oxygen desaturation index and excessive daytime sleepiness (EDS), with a mean improvement in the Epworth Sleepiness Scale (ESS) of -5 and a reduction in the apnea-hypopnea index (AHI) by 23 events per hour compared to conservative care. A systematic review and meta-analysis (113) found that bariatric surgery resulted in a 14 kg/m² decrease in BMI and a 29 events per hour reduction in AHI, compared to a 3.1 kg/m² and 11 events per hour decrease for non-surgical management. Another meta-analysis(114) of 2310 patients with OSA showed significant improvements with reductions in BMI by 11.9, AHI by 19.3, and respiratory disturbance index (RDI) by 33.9. Both reviews suggest that bariatric surgery is effective in reducing obesity and OSA severity, with additional benefits in reducing daytime sleepiness (measured by >50% statistically significant reduction in STOP BANG and ESS) and improving sleep architecture.(115)
Summary
- OSA affects almost 1 billion adults worldwide
- Awareness of the common risk factors and clinical presentation of OSA in primary care settings can improve patient health outcomes.
- Screening tools (eg OSA50, STOP-Bang and ESS) can help identify patients at high risk of symptomatic moderate–severe OSA.
- GP’s should ask about nasal obstruction in patients who complain of snoring and OSA
- GP’s should examine patients with snoring and sleep apnoea for anatomical causes of obstruction such as nasal blockage and enlarged tonsils
- Patients who meet MBS criteria can be directly referred by GPs for sleep study testing at an appropriate provider
- Sleep physician referral should be considered for patients with complex presentations or other suspected sleep disorders as well as heavy vehicle licence holders.
- Weight loss, reduced alcohol consumption and benzodiazepine/opioid cessation should be recommended, where appropriate for primary snoring and OSA
- CPAP therapy should be considered first line therapy for moderate – severe sleep apnoea
- MAS can be considered for OSA and should be an qualified dentist or orthodontist with appropriate training
- Patients should be referred to a sleep trained ENT surgeon if they have
- Nasal obstruction
- Snoring
- OSA with good anatomy for surgery or patients wanting a second opinion before commencing device therapy
- OSA patients failing or not able to tolerate device therapy (CPAP, MAS)
- Positional therapy should be considered in supine dominant snorers
- Bariatric surgery can be considered in obese patients (typically >35kg/m2)
- Referral to an Oral and Maxillofacial surgeon for OSA patients with maxillary or mandibular insufficiency for an opinion regarding MaxilloMandibular Advancement (MMA) can also be considered
References
1. Bearpark H, Elliott L, Grunstein R, Cullen S, Schneider H, Althaus W, et al. Snoring and sleep apnea: A population study in Australian men. Am J Respir Crit Care Med. 1995;151(5).
2. Li Y, Liu J, Wang W, Yong Q, Zhou G, Wang M, et al. Association of self-reported snoring with carotid artery intima-media thickness and plaque. J Sleep Res. 2012;21(1).
3. Kim J, Pack AI, Riegel BJ, Chirinos JA, Hanlon A, Lee SK, et al. Objective snoring time and carotid intima-media thickness in non-apneic female snorers. J Sleep Res. 2017;26(2).
4. Zou J, Song F, Xu H, Fu Y, Xia Y, Qian Y, et al. The relationship between simple snoring and metabolic syndrome: A cross-sectional study. J Diabetes Res. 2019;2019.
5. Marshall NS, Wong KKH, Cullen SRJ, Knuiman MW, Grunstein RR. Snoring is not associated with all-cause mortality, incident cardiovascular disease, or stroke in the busselton health study. Sleep. 2012;35(9).
6. Liu Y, Peng T, Zhang S, Tang K. The relationship between depression, daytime napping, daytime dysfunction, and snoring in 0.5 million Chinese populations: Exploring the effects of socio-economic status and age. BMC Public Health. 2018;18(1).
7. Blumen M, Quera Salva MA, D’Ortho MP, Leroux K, Audibert P, Fermanian C, et al. Effect of sleeping alone on sleep quality in female bed partners of snorers. European Respiratory Journal. 2009;34(5).
8. Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: The Sleep Heart Health Study. Arch Intern Med. 2002;162(8).
9. Benjafield A V., Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8).
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Vol. 165, American Journal of Respiratory and Critical Care Medicine. 2002.
11. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 Apr;177(9).
12. Durán J, Esnaola S, Rubio R, Iztueta Á. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 I).
13. Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6).
14. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of Sleep Apnea: Rationale, Design, and Major Findings of the Wisconsin Sleep Cohort Study. Medical Sciences Center. 2009 Aug;5(108):246–9.
15. Cunningham J, Hunter M, Budgeon C, Murray K, Knuiman M, Hui J, et al. The prevalence and comorbidities of obstructive sleep apnea in middle-aged men and women: The Busselton Healthy Ageing Study. Journal of Clinical Sleep Medicine. 2021;17(10).
16. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: THE HypnoLaus study. Lancet Respir Med. 2015 Apr 1;3(4):310–8.
17. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. Journal of Clinical Sleep Medicine. 2012;8(5).
18. Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40(14).
19. Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Javier Nieto F, et al. Sleep Disordered Breathing and Mortality: Eighteen-Year Follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31(8).
20. Marshall NS, Wong K, Liu P, Cullen S, Knuiman M, Grunsetin R. Sleep Apnea as an Independent Risk Factor for All-Cause Mortality: The Busselton Health Study. Sleep [Internet]. 2008;31(8):1079–85. Available from: https://academic.oup.com/sleep/article-abstract/31/8/1079/2454239
21. Watson NF. Health care savings: The economic value of diagnostic and therapeutic care for obstructive sleep apnea. Vol. 12, Journal of Clinical Sleep Medicine. 2016.
22. Deloitte Access Economics. Rise and try to shine: The social and economic cost of sleep disorders in Australia Sleep Health Foundation [Internet]. 2021 May. Available from: www.deloitte.com/au/deloitte-access-economics
23. Dong Z, Xu X, Wang C, Cartledge S, Maddison R, Shariful Islam SM. Association of overweight and obesity with obstructive sleep apnoea: A systematic review and meta-analysis. Obes Med. 2020;17.
24. Bonsignore MR, Saaresranta T, Riha RL, Riha R, Bonsignore M. Sex differences in obstructive sleep apnoea. European Respiratory Review. 2019;28(154).
25. Sweetman A, Lack L, McEvoy RD, Smith S, Eckert DJ, Osman A, et al. Bi-directional relationships between co-morbid insomnia and sleep apnea (COMISA). Vol. 60, Sleep Medicine Reviews. 2021.
26. Sweetman A. Co-morbid Insomnia and Sleep Apnoea (COMISA): Latest Research from an Emerging Field. Vol. 9, Current Sleep Medicine Reports. Springer Science and Business Media Deutschland GmbH; 2023. p. 180–9.
27. Yalamanchi P, Mott N, Ali SA, Peddireddy NS, Kovatch KJ, Stanley JJ, et al. Evaluation of In-Office Volitional Snore as a Screening Tool for Candidacy for Hypoglossal Nerve Stimulation. Otolaryngology – Head and Neck Surgery (United States). 2022;166(3).
28. Borowiecki BD, Sassin JF, Sassin JF. Surgical treatment of sleep apnea. Arch Otolaryngol [Internet]. 1983 Aug 1 [cited 2024 Dec 31];109(8):508–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6870642
29. Woodson BT, Feroah T, Connolly LA, Toohill RJ. A method to evaluate upper airway mechanics following intervention in snorers. American Journal of Otolaryngology – Head and Neck Medicine and Surgery. 1997;18(5).
30. Jugé L, Yeung J, Knapman FL, Burke PGR, Lowth AB, Gan KZC, et al. Influence of mandibular advancement on tongue dilatory movement during wakefulness and how this is related to oral appliance therapy outcome for obstructive sleep apnea. Sleep. 2021;44(3).
31. Chai-Coetzer CL, Antic NA, Rowland LS, Catcheside PG, Esterman A, Reed RL, et al. A simplified model of screening questionnaire and home monitoring for obstructive sleep apnoea in primary care. Thorax. 2011;66(3).
32. Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5).
33. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7).
34. Li Chai-Coetzer C, Hancock K. Diagnosis of obstructive sleep apnoea in primary care. AJGP. 2024 Jun;53(6).
35. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. Journal of Clinical Sleep Medicine. 2022 Apr 1;18(4):1093–102.
36. Carneiro-Barrera A, Díaz-Román A, Guillén-Riquelme A, Buela-Casal G. Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: Systematic review and meta-analysis. Vol. 20, Obesity Reviews. 2019.
37. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23).
38. Joosten SA, Hamilton GS, Naughton MT. Impact of Weight Loss Management in OSA. Vol. 152, Chest. 2017.
39. Kuna ST, Reboussin DM, Strotmeyer ES, Millman RP, Zammit G, Walkup MP, et al. Effects of weight loss on obstructive sleep apnea severity ten-year results of the sleep AHEAD study. Am J Respir Crit Care Med. 2021;203(2).
40. Edwards BA, Bristow C, O’Driscoll DM, Wong AM, Ghazi L, Davidson ZE, et al. Assessing the impact of diet, exercise and the combination of the two as a treatment for OSA: A systematic review and meta-analysis. Vol. 24, Respirology. 2019.
41. Burgos-Sanchez C, Jones NN, Avillion M, Gibson SJ, Patel JA, Neighbors J, et al. Impact of Alcohol Consumption on Snoring and Sleep Apnea: A Systematic Review and Meta-analysis. Vol. 163, Otolaryngology – Head and Neck Surgery (United States). 2020.
42. Malhotra A, Grunstein RR, Fietze I, Weaver TE, Redline S, Azarbarzin A, et al. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. New England Journal of Medicine. 2024 Oct 3;
43. Sweetman A, Lack L, Catcheside PG, Antic NA, Smith S, Li Chai-Coetzer C, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: A randomized clinical trial. Sleep. 2019;42(12).
44. National Institute for Health and Care Excellence (NICE). Obstructive sleep apnoea/hypopnoea syndrome and obesity hypoventilation syndrome in over 16s. 2021 Aug.
45. Patil SP, Ayappa IA, Caples SM, John Kimoff R, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: An American academy of sleep medicine clinical practice guideline. Journal of Clinical Sleep Medicine. 2019;15(2):335–43.
46. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. New England Journal of Medicine. 2016 Sep 8;375(10):919–31.
47. Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, Abad J, Duran-Cantolla J, Cabriada V, et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8(4).
48. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5).
49. Guzman MA, Sgambati FP, Pho H, Arias RS, Hawks EM. The efficacy of low-level CPAP for the treatment of snoring. J Clin Sleep Medleep. 2017;13(5).
50. Kulkas A, Leppänen T, Nikkonen S, Oksenberg A, Duce B, Mervaala E, et al. Required CPAP usage time to normalize AHI in obstructive sleep apnea patients: a simulation study. Physiol Meas. 2018 Nov 28;39(11):115009.
51. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc Am Thorac Soc. 2008 Feb;5(2):173–8.
52. Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: Implications for future interventions. Vol. 131, Indian Journal of Medical Research. 2010.
53. Ulander M, Johansson MS, Ewaldh AE, Svanborg E, Broström A. Side effects to continuous positive airway pressure treatment for obstructive sleep apnoea: Changes over time and association to adherence. Sleep and Breathing. 2014;18(4).
54. Zozula R, Rosen R, Phillips B. Compliance with continuous positive airway pressure therapy: Assessing and improving treatment outcomes. Vol. 7, Current Opinion in Pulmonary Medicine. 2001.
55. Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016 Aug 19;45(1):43.
56. Van Ryswyk E, Anderson CS, Antic NA, Barbe F, Bittencourt L, Freed R, et al. Predictors of long-term adherence to continuous positive airway pressure in patients with obstructive sleep apnea and cardiovascular disease. Sleep. 2019 Oct 1;42(10).
57. Ellender CM, Vakulin A, Stocks N, Li Chai-Coetzer C. Management of obstructive sleep apnoea in primary care. AJGP [Internet]. 2024 Jun;53(6). Available from: www.sleepprimarycareresources.org.au
58. Liu CY, Lu HY, Dong FS, Ma WS, Wang J, Hu XY, et al. Effects of a mandibular advancement device on genioglossus in obstructive sleep apnoea hypopnea syndrome. Eur J Orthod. 2015;37(3).
59. Ishida M, Inoue Y, Suto Y, Okamoto K, Ryoke K, Higami S, et al. Mechanism of action and therapeutic indication of prosthetic mandibular advancement in obstructive sleep apnea syndrome. In: Psychiatry and Clinical Neurosciences. 1998.
60. Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: Effect on awake calibre of the velopharynx. Thorax. 1999;54(11).
61. L’Estrange PR, Battagel JM, Harkness B, Spratley MH, Nolan PJ, Jorgensen GI. A method of studying adaptive changes of the oropharynx to variation in mandibular position in patients with obstructive sleep apnoea. J Oral Rehabil. 1996;23(10).
62. Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: An update for 2015. Journal of Clinical Sleep Medicine. 2015;11(7).
63. Vecchierini MF, Attali V, Collet JM, d’Ortho MP, Goutorbe F, Kerbrat JB, et al. Mandibular advancement device use in obstructive sleep apnea: ORCADES study 5-year follow-up data. Journal of Clinical Sleep Medicine. 2021 Aug 1;17(8):1695–705.
64. Robertson S, Murray M, Young D, Pilley R, Dempster J. A randomized crossover trial of conservative snoring treatments: Mandibular repositioning splint and nasal CPAP. Otolaryngology – Head and Neck Surgery. 2008;138(3).
65. Vanderveken OM, Devolder A, Marklund M, Boudewyns AN, Braem MJ, Okkerse W, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008;178(2).
66. Johal A, Hamoda MM, Almeida FR, Marklund M, Tallamraju H. The role of oral appliance therapy in obstructive sleep apnoea. Vol. 32, European Respiratory Review. European Respiratory Society; 2023.
67. Ravesloot MJL, White D, Heinzer R, Oksenberg A, Pépin JL. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: A systematic review of the literature and meta-Analysis. Vol. 13, Journal of Clinical Sleep Medicine. American Academy of Sleep Medicine; 2017. p. 813–24.
68. Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. Journal of Clinical Sleep Medicine. 2011;7(4).
69. Berry RB, Uhles ML, Abaluck BK, Winslow DH, Schweitzer PK, Gaskins RA, et al. Nightbalance sleep position treatment device versus auto-adjusting positive airway pressure for treatment of positional obstructive sleep apnea. Journal of Clinical Sleep Medicine. 2019;15(7):947–56.
70. Ferris B, Mead J, Opie L. Partitioning Of Respiratory Flow Resistance In Man. J Appl Physiol. 1964;19:653–8.
71. Georgalas C, Georgalas C, Georgalas C. The role of the nose in snoring and obstructive sleep apnoea: an update. Eur Arch Otorhinolaryngol [Internet]. 2011 Sep [cited 2025 Jan 30];268(9):1365–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21340561
72. Correa EJ, Conti DM, Moreno-Luna R, Sánchez-Gómez S, O’Connor Reina C. Role of Nasal Surgery in Adult Obstructive Sleep Apnea: A Systematic Review. Sleep Science. 2024 Sep 10;
73. Fitzpatrick MF, McLean H, Urton AM, Tan A, O’Donnell D, Driver HS. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur Respir J [Internet]. 2003 Nov [cited 2025 Jan 30];22(5):827–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14621092
74. 2020 Fokkens European Position Paper on Rhinosinusitis and Nasal Polyps 2020.
75. Bengtsson C, Lindberg E, Jonsson L, Holmström M, Sundbom F, Hedner J, et al. Chronic Rhinosinusitis Impairs Sleep Quality: Results of the GA2LEN Study. Sleep [Internet]. 2017 Jan 1 [cited 2025 Jan 31];40(1). Available from: http://www.ncbi.nlm.nih.gov/pubmed/28364469
76. Alt JA, Ramakrishnan VR, Platt MP, Schlosser RJ, Storck T, Soler ZM. Impact of chronic rhinosinusitis on sleep: a controlled clinical study. Int Forum Allergy Rhinol [Internet]. 2019 Jan [cited 2025 Jan 30];9(1):16–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30281930
77. Weaver TE. Don’t start celebrating – CPAP adherence remains a problem. Vol. 9, Journal of Clinical Sleep Medicine. 2013. p. 551–2.
78. Yetkin O, Kunter E, Gunen H. CPAP compliance in patients with obstructive sleep apnea syndrome. Sleep and Breathing. 2008;12(4):365–7.
79. Chen C, Wang J, Pang L, Wang Y, Ma G, Liao W. Telemonitor care helps CPAP compliance in patients with obstructive sleep apnea: a systemic review and meta-analysis of randomized controlled trials. Ther Adv Chronic Dis [Internet]. 2020 [cited 2025 Jan 26];11:2040622320901625. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32215196
80. Hussain SF, Irfan M, Waheed Z, Alam N, Mansoor S, Islam M. Compliance with continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea among privately paying patients- a cross sectional study. BMC Pulm Med [Internet]. 2014 Nov 29 [cited 2025 Jan 26];14(1):188. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25433468
81. Mehrtash M, Bakker JP, Ayas N, Ayas N. Predictors of Continuous Positive Airway Pressure Adherence in Patients with Obstructive Sleep Apnea. Lung [Internet]. 2019 Apr [cited 2025 Jan 26];197(2):115–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30617618
82. Inoue A, Chiba S, Matsuura K, Osafune H, Capasso R, Wada K. Nasal function and CPAP compliance. Auris Nasus Larynx [Internet]. 2019 Aug [cited 2025 Jan 30];46(4):548–58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30538069
83. Sugiura T, Noda A, Nakata S, Yasuda Y, Soga T, Miyata S, et al. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration [Internet]. 2007 [cited 2025 Jan 30];74(1):56–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16299414
84. McLean HA, Urton AM, Driver HS, Tan AKW, Day AG, Munt PW, et al. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur Respir J [Internet]. 2005 Mar [cited 2025 Jan 26];25(3):521–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15738298
85. Jiang RS, Liang KL, Liang KL. The Influence of Functional Endoscopic Sinus Surgery on Sleep Related Outcomes in Patients with Chronic Rhinosinusitis. Int J Otolaryngol [Internet]. 2019 [cited 2025 Jan 31];2019:7951045. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31275397
86. Camacho M, Riaz M, Capasso R, Ruoff CM, Guilleminault C, Kushida CA, et al. The effect of nasal surgery on continuous positive airway pressure device use and therapeutic treatment pressures: A systematic review and meta-analysis. Sleep. 2015 Feb 1;38(2):279-286A.
87. Kent D, Stanley J, Aurora RN, Levine C, Gottlieb DJ, Spann MD, et al. Referral of adults with obstructive sleep apnea for surgical consultation: an American Academy of Sleep Medicine clinical practice guideline. Vol. 17, Journal of Clinical Sleep Medicine. American Academy of Sleep Medicine; 2021. p. 2499–505.
88. Lindsay BM, Sarkis LM, Sideris AW, Lam ME, Jones A, MacKay SG. Modified uvulopalatopharyngoplasty and radiofrequency-in-saline tongue for the management of snoring. In: Australian Journal of Otolaryngology. AME Publishing Company; 2022.
89. Bonsignore MR, Lombardi C, Lombardo S, Fanfulla F. Epidemiology, Physiology and Clinical Approach to Sleepiness at the Wheel in OSA Patients: A Narrative Review. Vol. 11, Journal of Clinical Medicine. 2022.
90. Bioulac S, Micoulaud Franchi JA, Arnaud M, Sagaspe P, Moore N, Salvo F, et al. Risk of motor vehicle accidents related to sleepiness at the wheel: A systematic review and meta-analysis. Vol. 40, Sleep. 2017.
91. Sina EM, Shankar S, Boon MS, Huntley CT. Risk of Motor Vehicle Accidents in Obstructive Sleep Apnea: Comparative Analysis of CPAP Versus Surgery. Otolaryngology – Head and Neck Surgery (United States). 2025;
92. Howard ME, Desai A V., Grunstein RR, Hukins C, Armstrong JG, Joffe D, et al. Sleepiness, sleep-disordered breathing, and accident risk factors in commercial vehicle drivers. Am J Respir Crit Care Med. 2004;170(9).
93. Assessing fitness to drive for commercial and private vehicle drivers 2 0 2 2 E D I T I O N Medical standards for licensing and clinical management guidelines [Internet]. 2022. Available from: www.austroads.com.au
94. Gurubhagavatula I, Sullivan S, Meoli A, Patil S, Olson R, Berneking M, et al. Management of obstructive sleep apnea in commercial motor vehicle operators: Recommendations of the AASM sleep and transportation safety awareness task force. Journal of Clinical Sleep Medicine. 2017;13(5).
95. Hellings P, Jorissen M, Ceuppens JL. The Waldeyer’s ring. Acta Otorhinolaryngol Belg. 2000;54(3).
96. Guimaraes CVA, Kalra M, Donnelly LF, Shott SR, Fitz K, Singla S, et al. The frequency of lingual tonsil enlargement in obese children. American Journal of Roentgenology. 2008;190(4).
97. Sung MW, Lee WH, Wee JH, Lee CH, Kim E, Kim JW. Factors associated with hypertrophy of the lingual tonsils in adults with sleep-disordered breathing. JAMA Otolaryngol Head Neck Surg. 2013;139(6).
98. Friedman M, Wilson MN, Pulver TM, Golbin D, Lee GP, Gorelick G, et al. Measurements of adult lingual tonsil tissue in health and disease. Otolaryngology – Head and Neck Surgery. 2010;142(4).
99. Vroegop A V., Vanderveken OM, Boudewyns AN, Scholman J, Saldien V, Wouters K, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: Report on 1,249 cases. In: Laryngoscope. 2014. p. 797–802.
100. Lan WC, Chang WD, Tsai MH, Tsou YA. Trans-oral robotic surgery versus coblation tongue base reduction for obstructive sleep apnea syndrome. PeerJ. 2019;2019(10).
101. Murphey AW, Kandl JA, Nguyen SA, Weber AC, Gillespie MB. The Effect of Glossectomy for Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. In: Otolaryngology – Head and Neck Surgery (United States). SAGE Publications Inc.; 2015. p. 334–42.
102. Woodson BT, Strohl KP, Soose RJ, Gillespie MB, Maurer JT, de Vries N, et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngology – Head and Neck Surgery (United States). 2018 Jul 1;159(1):194–202.
103. Suurna M V., Steffen A, Boon M, Chio E, Copper M, Patil RD, et al. Impact of Body Mass Index and Discomfort on Upper Airway Stimulation: ADHERE Registry 2020 Update. Laryngoscope. 2021;131(11).
104. Schwartz AR, Jacobowitz O, Eisele DW, Mickelson SA, Miller MB, Oliven A, et al. Targeted Hypoglossal Nerve Stimulation for Patients with Obstructive Sleep Apnea: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2023 Jun 8;149(6):512–20.
105. Thaler E, Schwab R, Maurer J, Soose R, Larsen C, Stevens S, et al. Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy. Laryngoscope. 2020 May 14;130(5):1333–8.
106. Yu PK, Stenerson M, Ishman SL, Shott SR, Raol N, Soose RJ, et al. Evaluation of Upper Airway Stimulation for Adolescents With Down Syndrome and Obstructive Sleep Apnea. JAMA Otolaryngol Head Neck Surg. 2022 Jun 1;148(6):522–8.
107. Chieffe D, Liu RH, Hartnick C. Challenges and adverse events in pediatric hypoglossal nerve stimulation. Int J Pediatr Otorhinolaryngol. 2024 Jan 1;176.
108. Kent DT, Zealear D, Schwartz AR. Ansa Cervicalis Stimulation: A New Direction in Neurostimulation for OSA. Chest. 2021 Mar 1;159(3):1212–21.
109. Oh DM, Johnson J, Shah B, Bhat S, Nuoman R, Ming X. Treatment of vagus nerve stimulator-induced sleep-disordered breathing: A case series. Epilepsy Behav Rep. 2019;12.
110. Marzec M, Edwards J, Sagher O, Fromes G, Malow BA. Effects of vagus nerve stimulation on sleep-related breathing in epilepsy patients. Epilepsia. 2003;44(7).
111. Iftikhar MH, Darken R. OSA: A CONSEQUENCE OF VAGAL NERVE STIMULATION. Chest. 2020;158(4).
112. Hudgel DW, Patel SR, Ahasic AM, Bartlett SJ, Bessesen DH, Coaker MA, et al. The role of weight management in the treatment of adult obstructive sleep apnea: An official American thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2018;198(6).
113. Ashrafian H, Toma T, Rowland SP, Harling L, Tan A, Efthimiou E, et al. Bariatric Surgery or Non-Surgical Weight Loss for Obstructive Sleep Apnoea? A Systematic Review and Comparison of Meta-analyses. Obes Surg. 2015 Jul 25;25(7):1239–50.
114. Al Oweidat K, Toubasi AA, Tawileh RBA, Tawileh HBA, Hasuneh MM. Bariatric surgery and obstructive sleep apnea: a systematic review and meta-analysis. Sleep and Breathing. 2023 May 5;
115. Nastałek P, Polok K, Celejewska-Wójcik N, Kania A, Sładek K, Małczak P, et al. Impact of bariatric surgery on obstructive sleep apnea severity and continuous positive airway pressure therapy compliance—prospective observational study. Sci Rep. 2021 Dec 1;11(1).